Since the onset of the Covid-19 virus, the development of vaccines and therapeutics has been going full speed. (Reference our previous article for a glance at the development of treatment during the early days of the virus outbreak.) At this point, the FDA has approved a vaccine, treatments, and has authorized vaccine boosters. Agreements among companies are one way to take a look at the innovation occurring within the industry. Here at ktMINE, we have a daily batch of fresh agreements individually analyzed to pick through, with no lack of those related to Covid-19. So, let’s take a look at what’s occurring in a few of these agreements.
2020 Agreements
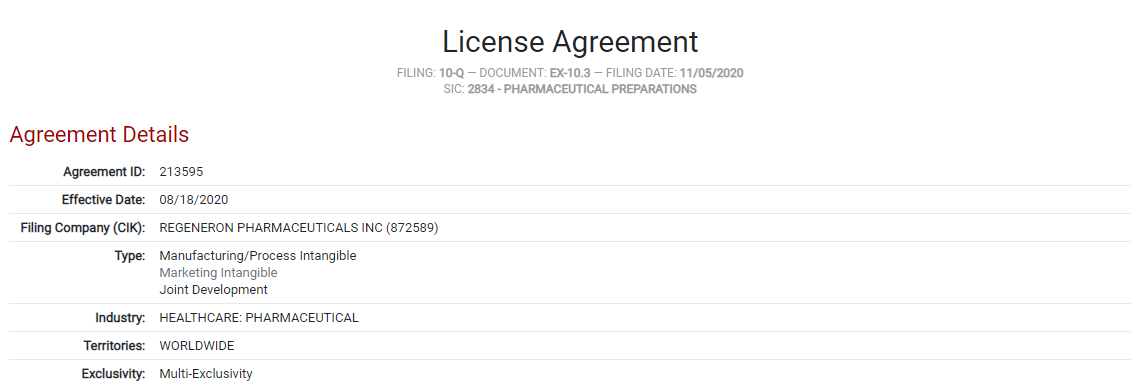
One of the treatments authorized by the FDA for emergency use is Regen-Cov, an antibody “cocktail” made by Regeneron Pharmaceuticals Inc. This therapy was recently in the news due to an agreement with the U.S. government to purchase its second supply of Regen-cov. Document ID 213595 in the ktMINE database refers to the licensing of what is an “antibody cocktail” made up of compounds called REGN10933 and REGN10987 as a treatment for Covid-19. In the agreement, Regeneron grants manufacturing and marketing rights of this proprietary cocktail to F. Hoffmann-La Roche Ltd. and its subsidiary Genentech, Inc. in every country but the United States (this territory is retained by Regeneron). This agreement went into effect in August of 2020.
Generex Biotechnology Corp has engaged in quite a few agreements related to its Ii-Key Peptide Vaccine technology for Covid-19. The earliest being a collaboration from February of 2020 (ID 210355) with Beijing Zhonghua Investment Fund Management Co., LTD. and Sinotek-Advocates International Industry Development (Shenzhen) Co., Ltd. II-Key is defined in the agreement as an “immunoregulatory protein.” The focus of the technology is to use the Covid-19 spike protein fragments to stimulate a response from T-helper cells which plays a major role in helping the body protect itself through the development of antibodies. Generex granted a range of rights to this Ii-Key technology to Zhoungha and Sinotek in exchange for the provision of the means to evaluate the efficacy and commercialibility of the vaccine in China as well as an upfront payment in the millions, and a royalty.
Another agreement (ID 228354) related to the Ii-Key vaccine was formed later that year in November of 2020. Although this agreement does not explicitly mention Covid-19, it was stated to be a potential target of the vaccine in a press release filed by Generex. This agreement is regarding the development of the Ii-Key vaccine by Generex’s subsidiary, NuGenerex Immuno-Oncology Inc., and their partners: Beijing Youfeng International Consulting Co., Ltd.; Beijing Guoxin Haixiang Equity Investment Partnership; and National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention. In this agreement, all parties are contributing to the formation of a Joint Venture to work on the development of the Ii-Key vaccine in order to obtain regulatory approval in China. This agreement contains a license fee paid by the Joint Entity to NuGenerex.
2021 Agreement

Though there are many more Covid-19 related agreements from 2020 to discuss, in the ktMINEdatabase is also agreement 213978 filed this year by NantKwest, Inc., although it has an effective date in August of 2020. Here, a United Kingdom company, Stabilitech Biopharma Ltd., is exclusively licensing to ImmunityBio, Inc., based in the United States, its proprietary Covid-19 treatment. The product “proprietary OraPro-COVID-19 vaccine product” is defined as “a viral vectored adenovirus 5 containing the spike protein DNA from the COVID-19 virus.” The license also allows ImmunityBio to use their own “coronavirus vaccine candidates, including hAd5 [adenovirus constructs]” to incorporate with Stabilitech’s intellectual property related to the licensed vaccine. The use of the product is limited “for the prevention and treatment of SARS-COV-2 and coronavirus successors” worldwide.
With a continuing worldwide need for treatments for Covid-19 and its variants, it’s no surprise that there are several agreements surrounding the virus. As the need for effective and alternative treatments continues, so will innovation through intellectual property.