Around 14 percent of the American population experience mental health issues in a given year. In a search of patents related to pharmaceuticals within the ktMINE platform, including keywords related to mental health disorders, show that the number of filed and granted patents has been on the rise. The pharmaceutical industry has also been growing, having a global value of about 1 trillion dollars. The United States (U.S.) accounts for almost 46% of the pharmaceutical industry’s revenue. This dominance of the U.S. can also be found in the analytics, reflected in the graphs below retrieved from the same patent search. Products that treat health issues almost guarantee a return on investments thanks to patents granted by the USPTO and market exclusivity approved by the FDA (Food and Drug Administration). Let’s examine the origin of the pharmaceutical industry with a focus on a division that’s increasingly being utilized by Americans now than ever before: psychiatry.
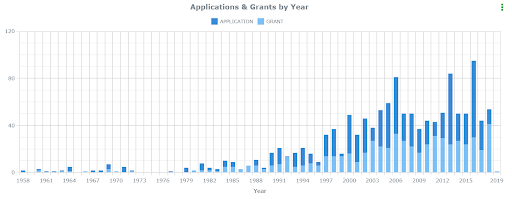
Patents included keywords: mental illness or psychiatry; and treatment and pharmaceuticals
Early Medicine
In 1790, patents started to be filed and granted in the U.S. It wasn’t until six years later that the “Bilious Pills” became the first drug to be patented. This drug was marketed as a treatment for a broad array of ailments from scurvy to jaundice (making broad, unfounded claims was a common practice for medicine). Patented medicine at this time referred to all drugs which the government granted and protected as exclusive. Even after the first patented medicine, research for mental illness did not make progress until over a century later. Popular remedies to mental illness in the 19th century were “home” remedies such as morphine, opium, or cocaine. In the late 19th and even into the 20th century, more immediate solutions rose in use such as lobotomies, electroshock/electroconvulsive treatment, and institutionalization, particularly for depression and schizophrenia.
It wasn’t until the mid 20th century that thought was given into researching mental health treatments. In 1950, it was observed that patients being treated for tuberculosis were having positive side effects such as euphoria, psychostimulation, increased appetite, and improved sleep; all which those with depression lack. Upon testing, the tuberculosis medication, iproniazid, was shown to make a major improvement in 70% of depressed patients and became the first unofficial treatment for depression. This accidental realization marked the beginning of active research in the field of psychiatry.
The Breakthrough via Schizophrenia
In 1951, chlorpromazine was chemically developed in France as a form of anesthesia. Over the next year, various compounds were tested on patients suffering from mania and schizophrenia. In 1952, the drug was proven to be an effective treatment and became available for prescription in France. That same year, Smith-Kline & French purchased the rights to the drug and upon FDA approval in 1954, began to market it as Thorazine. Throughout the next few years, as chlorpromazine studies were released, the drug spread and became increasingly utilized throughout the world. Chlorpromazine became the first marketed neuroleptic or antipsychotic and the first antipsychotic drug approved by the FDA for psychiatric treatment. The generic version of this drug came to the U.S. in 1973, even though by the late 1960s the popularity of the drug fell off with the rise of antidepressants.
From Anxiety to Depression: The Focus of Psychiatry
During the rise of chlorpromazine, anxiety was the focus of discussion around mental health. This was so much so that in 1962, anxiety was measured as the most commonly diagnosed illness. As a result, numerous drugs of the 1950s and 60s were directed toward the treatment of anxiety. Its popularity was likely inflated due to anxiety being vaguely defined with terms such as “stress” and “neurosis.” The first largely distributed treatment for anxiety was meprobamate. Meprobamate (named Miltown) grew to become the most popular prescription drug in U.S. history. Benzodiazepine Librium eventually replaced Miltown, and Valium was ultimately the most prescribed drug on the market.

The oldest meprobamate patent for anxiety in the ktMINE database
After the 70s, depression steadily rose so much that by 1975, it officially overtook anxiety in the number of diagnosed. A major factor that played into this shift was the approach taken at redefining the meaning of anxiety which dismantled it into separate disorders. More significantly, depression had more biological support, thus was able to further legitimize the field of psychiatry. Though not the focus, drugs for depression were also discovered during this psychiatric breakthrough. In 1955 imipramine was discovered during research for schizophrenia and was one of the very first antidepressants on the market as Tofranil. The generic alternative of imipramine started being marketed in the U.S. in 1976. This patent is available on the ktMINE database and can be seen below. Fluoxetine was the first SSRI (serotonin reuptake inhibitor, a class of antidepressants) marketed as Prozac in 1986.

The original imipramine patent in the ktMINE database
Exclusivity vs Patents
Exclusivity is the main incentive in applying for a patent and can be attained for drugs from either the FDA or the USPTO (U.S. Patent Office). The major differences between the two are the time of exclusivity and the application process. Patents are beneficial due to a longer exclusivity period of twenty years versus the maximum of twelve years granted by the FDA. The application for FDA approval is also more strenuous. The amount of testing and clinical trials that the FDA requires not only makes the process of obtaining FDA approval longer but the cost is astronomical, averaging out to billions of dollars for successful applications. Even in light of this, one path toward exclusivity should not be chosen over the other. Both are helpful in establishing the incentive to research and develop. This is evident considering that the U.S. has been the global leader in granting New Drug Patents, and the FDA is also increasingly approving more new molecular entities.
Strategies for using Drug Patents
There are ways patents can be used to advance drugs in the market. One lies in the exclusivity type. Unlike patents, FDA approval is required for any drug to be marketed in the U.S. and essentially required in order to receive any business from health insurers, who represent a majority of prescription drug spending. Because of this, it’s tempting to think that exclusivity through the FDA is all that’s really needed. However, the FDA approval process is the area that entails the majority of the costs of marketing a new drug, and gaining exclusivity is dependant on approved (which is criticized to be based on unstably enforced standards). As for patents, they are able to be applied for during any stage of the drug development. Thus, patenting early in the development process is protection for the investment. While a patent isn’t necessarily needed to sell a drug, it can help.
The other strategy to increase the profit of a drug involves extending the life of exclusivity since it is the main source of competition against generic drugs (drugs produced based on an expired patent or exclusivity period); especially due to the actual cost of making the drug being minuscule (usually mere pennies). One way of expanding a patent is by reformulating the molecular structure of the drug in order to qualify it as a new product. For example, Prozac, an SSRI, was altered from being a daily to being a weekly dosage. Another way to extend exclusivity is by applying to change the marketing direction of the drug which can be done through reformulation. An example of this is Paxil, an SSRI, being marketed to include it as a treatment for generalized anxiety disorder in 2001.
Evolution of Main Patent Owners
Then: Historically, discoveries were not made in the biggest pharmaceutical laboratories, especially since a lot of the initial psychiatric breakthroughs were achieved somewhat accidentally. Chlorpromazine was synthesized during research related to nausea and allergies. And the first antidepressant was created by a man in Switzerland after many tests that would be considered unorganized by today’s standard. Using the search ran earlier on the growth of psychiatric-related patents, I can then group the results by patent owners. I edited my result to include only those from the beginning of the psychiatric breakthrough, the 1950s and onward to 1990. The resulting top ultimate parents owners were MERCK & CO INC, PFIZER INC, NOVO NORDISK AS, and ASTRAZENECA PLC. AstraZeneca only owned 4 patents and is the only company that will not appear in the current top patent holders. Most of their patents were for molecules treating general “mental disorders,” although a few did specify depression, psychosis, and anti-aggressive behavior. AstraZeneca had one targeting more specific diseases such as Senile Dysfunction and Huntington’s.
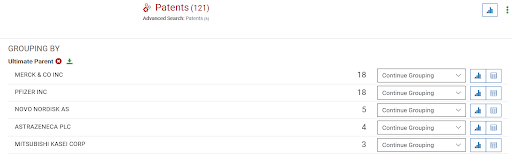
Top ultimate parent, psychiatric patent holders in the late 1900s
Now: So many discoveries in medicine have been made that it seems like we may be running out. It was found that as much as 80% of newly filed patents for drugs are regarding drugs which already existed on the market. This is a result of companies protecting older drugs, most likely using one of the strategies above. This sheds light on why the top pharmaceutical companies have not changed much from decades ago. However, this lack of discovery must be the case in the more general field of pharmaceuticals as it contradicts with the increase in the variety of the patents found in the more recent patent owners. The current, top ultimate parent of patent owners in the realm of pharmaceuticals related to mental disorder are PFIZER INC, OTSUKA PHARMACEUTICAL CO LTD, MERCK & CO INC, JOHNSON & JOHNSON, and NOVO NORDISK AS. One change in the trend of the more recent patents is the specificity of the treatment. Pfizer Inc’s. most recent patents evolved from treating “general” mental disorder to treatment ranging from learning/mental disorders associated with age, psychiatric and ocular disorders, and addictions. Merck & Co Inc.’s older patents date back to the late 60s and were related to general mental disorders, or senile dysfunction and now have patents which treat a wide variety mental disorders like eating disorders and attention deficit disorder.
The Future
The pharmaceutical industry is one which flourished from the start of medicine patents and does not seem to be slowing down anytime soon. The industry is expected to have growth of over 100% in every major country worldwide. While many historical diseases have been controlled or even eradicated, mental illness is one which is not wavering. However, this prevalence is not necessarily due to an increased amount of people who have a mental illness. It has been suggested that the increase in readily available psychiatric treatment has influenced more people to take advantage of it. Though, the most significant factor is said to be the overall increase in life expectancy resulting in a higher prevalence of ailments such as mental illness. For whatever reason it may be, the prevalence of mental illness is supported by the role of psychiatry in pharmaceutical industry revenue. Psycho-pharmaceuticals alone is responsible for almost $15 billion dollars. As stated initially, this is particularly the case in the American population which consumes the majority of the psychiatric pharmaceuticals in the world. While mental illness may not be increasing overall, for those who do live with it, the availability and advancement of treatment are important, and the guaranteed exclusivity of pharmaceutical discoveries granted by patents and the FDA has shown to be a positive force in promoting discovery in the industry.