On July 24th, the Samsung joint venture project, Samsung Bioepis, announced that they have begun to sell their Remicade biosimilar drug Renflexis in the United States. Remicade is widely used in treatment for autoimmune disorders from rheumatoid arthritis to Crohn’s Disease. It brought owners Johnson & Johnson sales of $4.8 billion last year in the US alone. After Pfizer released its Remicade biosimilar Inflectra late last year, Johnson & Johnson saw sales of Remicade fall by 8.2% in the first quarter of 2017. With Renflexis priced at a 35% discount of Remicade, the Samsung joint venture with Merck is poised to further disturb Johnson & Johnson’s hold on autoimmune treatment in the US.
Samsung Expands Its Pharma IP Portfolio
Bioepis is not Samsung’s first venture into the territory of biotechnology inventions, and it will certainly not be its last. In the last twenty years, Samsung has begun to develop its pharmaceutical patent portfolio. At present, Samsung Electronics Company Limited, the ultimate Samsung parent, has 211 patents, with the majority of its pharmaceutical patents being granted in the last 18 months.
Chart 1. Samsung’s Pharma IP Trends – Patent Application and Grant for Past 20 Years
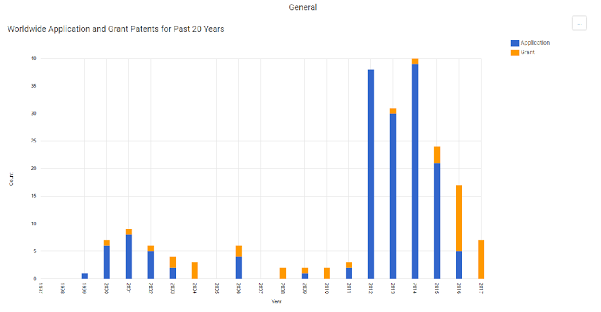
From ktMINE Search: Ultimate Parent “Samsung Electric Co Ltd” + Full Text “pharmaceutical”
The company has received the majority of its pharmaceutical patents in the United States and South Korea.
Chart 2. Samsung’s Top Filing Countries of Pharma Patents
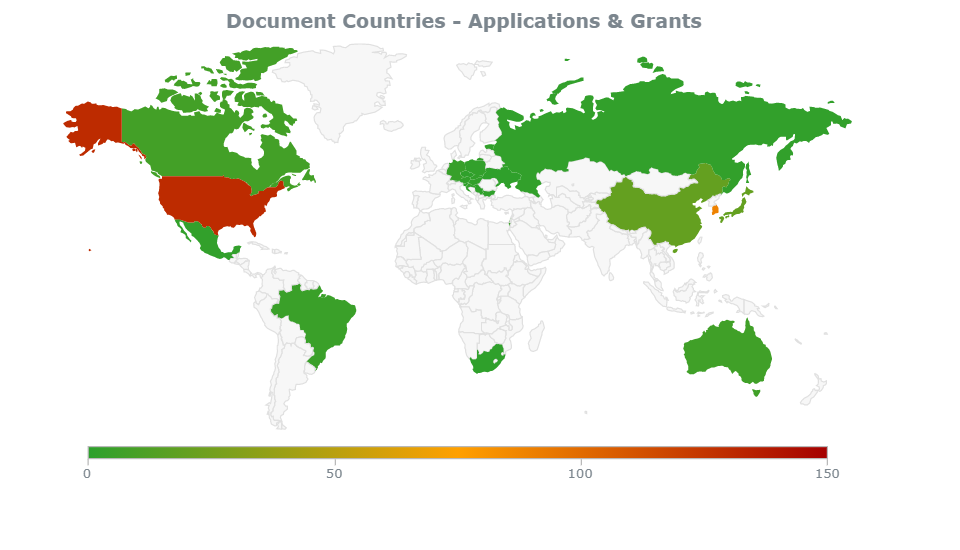
From ktMINE Search: Ultimate Parent “Samsung Electric Co Ltd” + Full Text “pharmaceutical”
While Samsung nominally expanded into pharmaceuticals in 2011 with the creation of the Samsung Biologics Company, it entered its first pharmaceutical patent application as far back as 1999. The company’s plan to break into the pharmaceutical game has been a long time coming. With its $740 million biologics manufacturing plant in Incheon, South Korea, receiving permission by the FDA to produce pharmaceuticals for the US market, Samsung has every intention of developing its biotechnology and biopharmaceutical subsidiary into a major pharmaceutical player worldwide.
Samsung Biosimilars
The focus of Samsung’s pharmaceutical business will be twofold. First, Samsung Bioepis will continue to develop biosimilars to name-brand drugs already on the market. These biosimilars will be priced at a discount to entice consumers to switch their drug therapies to Samsung’s less expensive product. Currently, Samsung Bioepis has four biosimilars in addition to Renflexis awaiting regulatory approval, and one in its last clinical trial phase:
- Etanercept, a biosimilar of Enbrel, used to treat rheumatoid arthritis and plaque psoriasis
- Adalimumab, a biosimilar of Humira, used to treat arthritis, plaque psoriasis, Crohn’s disease, and ulcerative colitis
- Insulin Glargine, a biosimilar of Lantus, used to treat diabetes
- Trastuzumab, a biosimilar of Herceptin, used to treat breast, stomach, and esophageal cancer
- Bevacizumab, a biosimilar of Avastin, used to treat colorectal, lung, ovarian, and cervical cancer
Samsung Biologics
Second, Samsung BioLogics will look to develop proprietary biologics based upon the pharmaceutical patents that Samsung already owns. Patents owned by Samsung give evidence of the kinds of drugs it will look to produce in the coming years. Considering Samsung’s patent portfolio related to biotechnology and pharmaceuticals, the focus on peptides, micro-organisms, and enzymes, as seen below in Chart 3, signals Samsung’s strong focus on the biosimilars market as opposed to chemically derived pharmaceuticals.
Some of Samsung’s most recent pharmaceutical patent applications and grants include:
- Composition for Preventing or Treating Muscle Wasting-Related Disease and Use Thereof (US9687498B1)
- Liposome Comprising Elastin-Like Polypeptide and Tumor Cell Targeting Material and Use Thereof (US2017165198A9)
- Composition for Promoting Remyelination in Nerve Cells Comprising 2,5-Dihydroxybenzenesulfonic Acid and Use Thereof (US2017049730A1)
- P15 Protein Variant and Use Thereof for Preventing or Treating Cancer (US9512193B2)
- Composition for Preventing or Treating Side Effect of Steroid in Subject Comprising Acetylsalicylic Acid and Use Thereof (US9492464B2)
Renflexis is only the tip of the iceberg; Samsung’s entry into the US pharmaceutical market is a portent to what is to come from the South Korean giant as it continues to invest in and develop its biopharma IP to become a major player in pharmaceutical business worldwide.
Chart 3. Samsung Patent Portfolio in Pharma – Top 10 CPC Codes
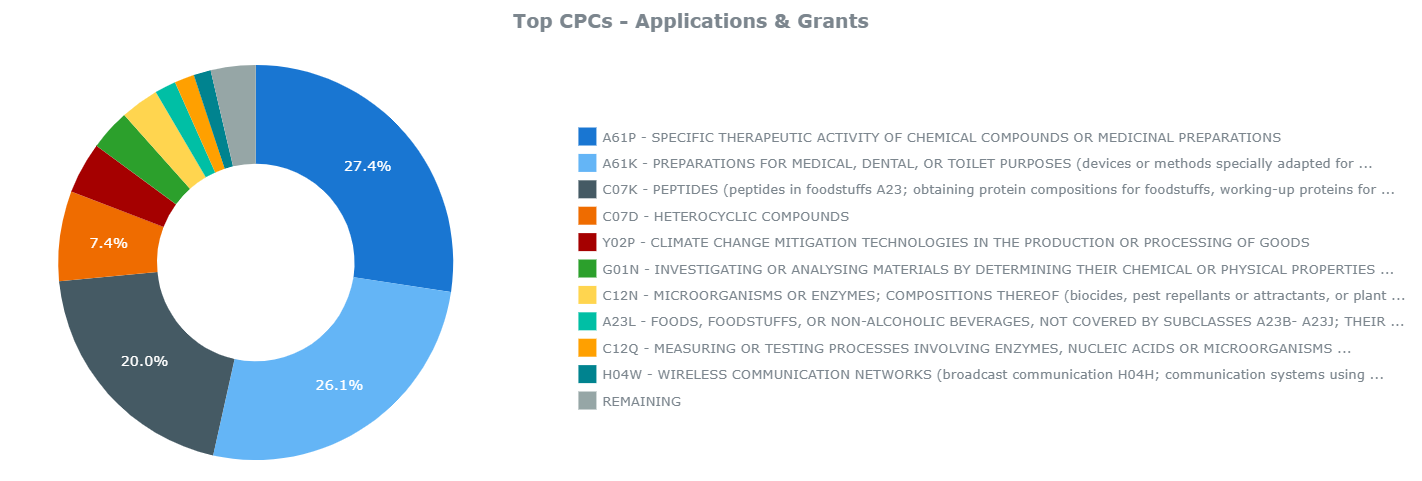
From ktMINE Search: Ultimate Parent “Samsung Electric Co Ltd” + Full Text “pharmaceutical”