This is the third installment in our series highlighting the different areas to investigate in an effort to strengthen your company’s IP research strategy. In parts one and two, we discussed the importance of analyzing emerging technologies as well as transacted technologies. In this post, we shift gears a little and talk about licensed technologies.
Companies often license-in technologies due to a lack of in-house development resources and acquisition opportunities. Having a pulse on licensing activity provides companies insight into competitors’ relevant technology areas of interest.
Leiden University and Bellicum Background
Leiden University is one of Europe’s leading international research universities focusing on key scientific fields including Life Sciences, Health, and Wellbeing. The university was founded back in 1575 and is located in Leiden, Netherlands. Leiden University has more than 6,700 employees and 29,520 students.
On the other hand, Bellicum is a pharmaceutical startup founded in 2004 and headquartered in Houston, Texas. It is a clinical-stage company focused on developing novel cellular immunotherapies for certain cancers and orphan inherited blood disorders. Bellicum currently has 173 employees.
Bellicum’s CaspaCIDe Technology: “CID Safety Switch”
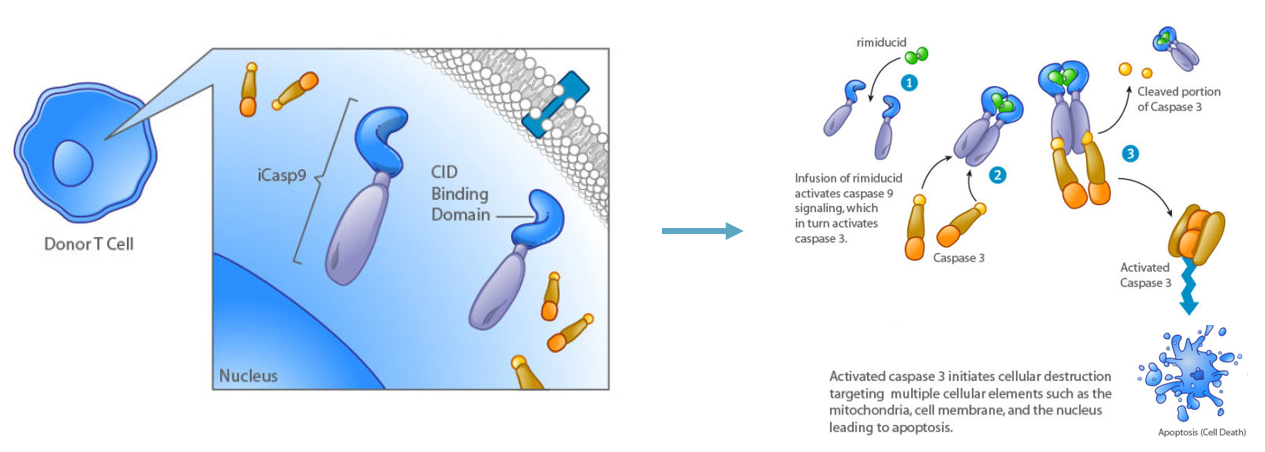
Source: Bellicum
More specifically, Bellicum has developed a patented “safety switch” technology called “CaspaCIDe” which addresses issues related to transplanted tissues attacking the host.
So, where does Leiden fit?
The next step for Bellicum in commercializing its technology is identifying use-cases for which its technology is best suited. As it turns out, Leiden’s Department of Hematology develops natural high-affinity T-cell receptors or “TCRs”, which are engineered T-cells that become activated in the presence of cancer cells. Certainly, Bellicum could expand efforts to develop and curate a list of candidates, but this work lies outside their area of expertise. With that said, Bellicum entered into a license agreement with Leiden University Medical Center, for worldwide rights to develop, manufacture and commercialize high-affinity TCR product candidates targeting solid tumors expressing the preferentially-expressed antigen in melanoma, or PRAME. BPX-701 was the first T-cell that was licensed by Bellicum from Leiden back in 2015.
Leiden University IP Profile
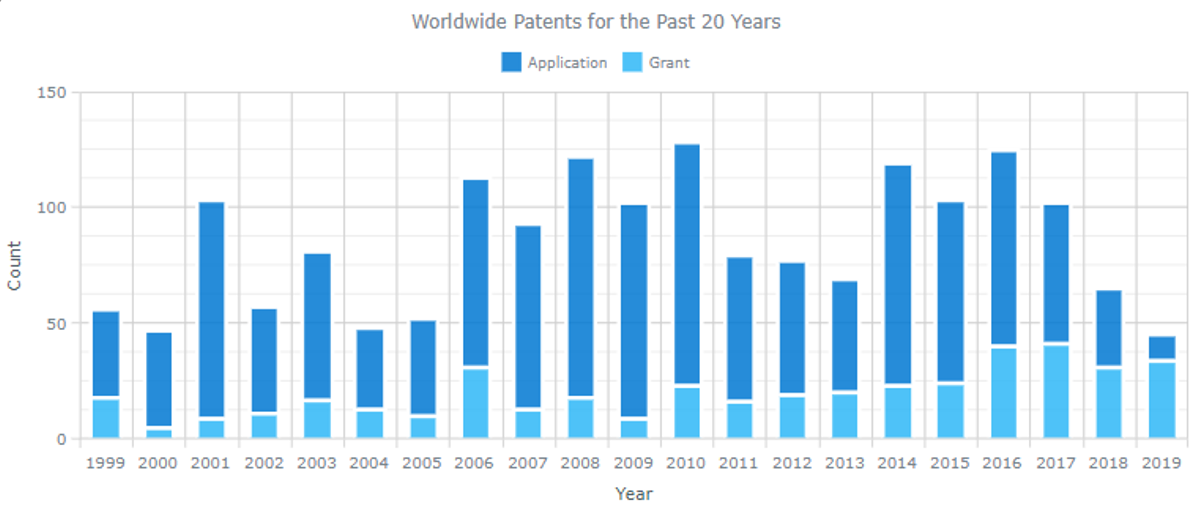
Source: ktMINE IP Platform
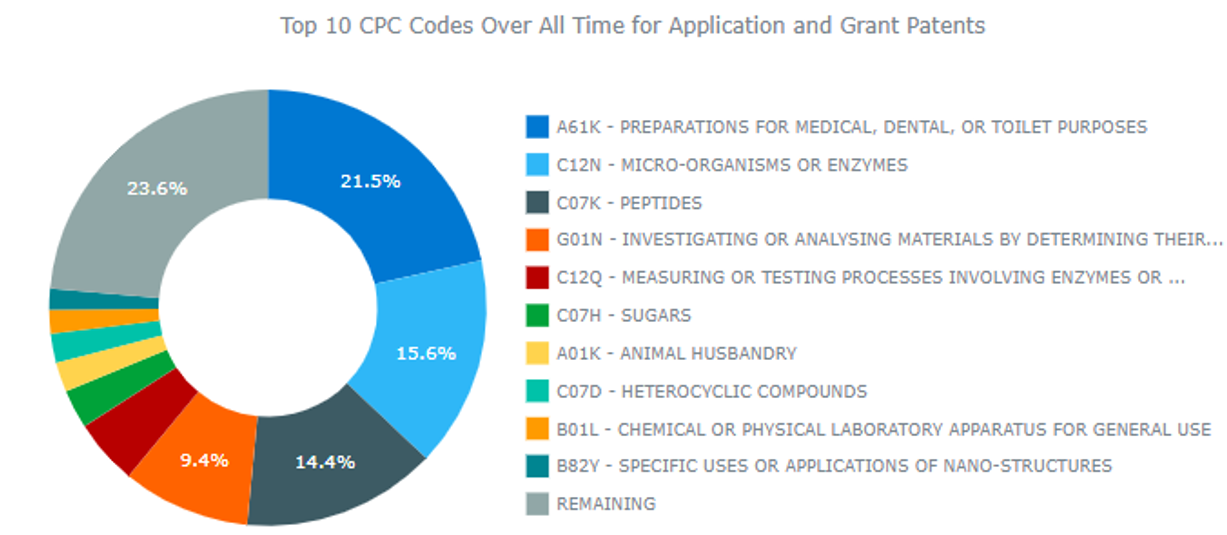
Source: ktMINE IP Platform
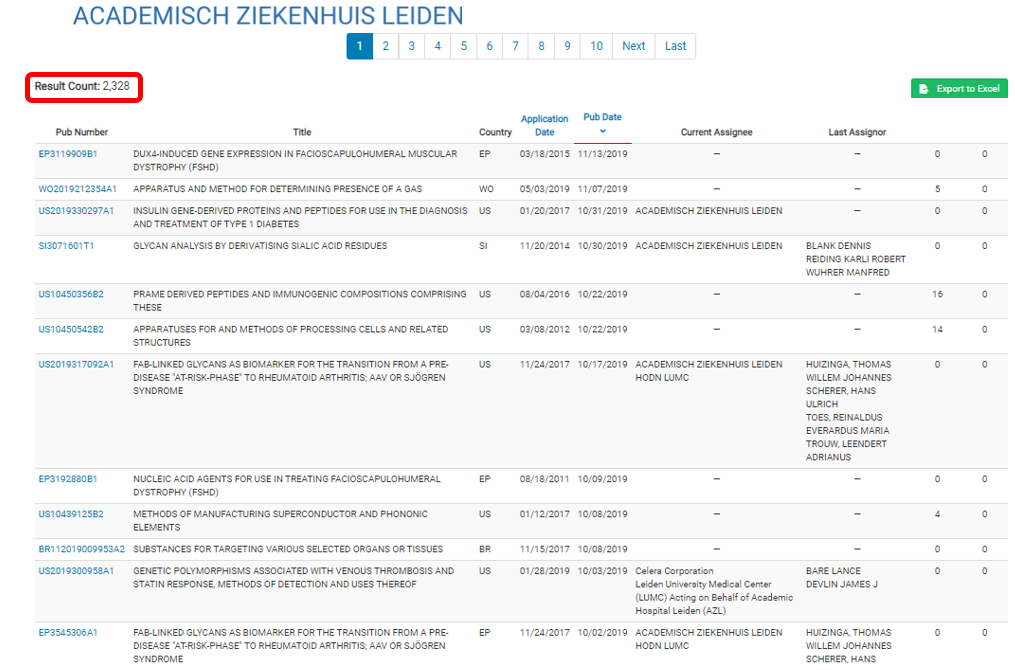
Source: ktMINE IP Platform
The above bar chart shows Leiden’s filing rates and the about pie chart indicates the innovation areas that Leiden is active in. It is likely that the researchers at Bellicum observed that Leiden’s progression was congruent with Bellicum’s need for a source of T-cells, and concluded that Leiden would be a good fit for Bellicum.
Sponsored Research Agreement

Source: ktMINE IP Platform
The above agreement shows that Bellicum provides financial support over a set period of time in exchange for the exclusive license to the technology and TCRs discovered.
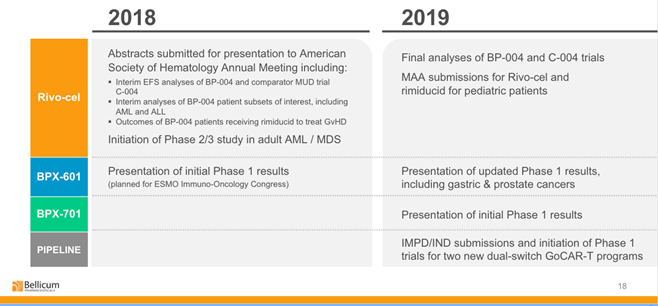
Source: Bellicum
The table above shows the progress enabled by the agreement. Ultimately, the license from Leiden is translating into solutions Bellicum can monetize.
Bellicum and Leiden University
How are both Bellicum and Leiden stronger? With the agreement, Bellicum can focus its efforts rather than “boil the ocean” or “straddle”, while Leiden University is incentivized to continue research in their space, enabling Bellicum to continue their efforts. This is an example of leveraging time-shifting for a faster time-to-market. It is a win-win-win – for Bellicum, Leiden University, and those benefiting from the resulting products.